TNKase approved for stroke treatment
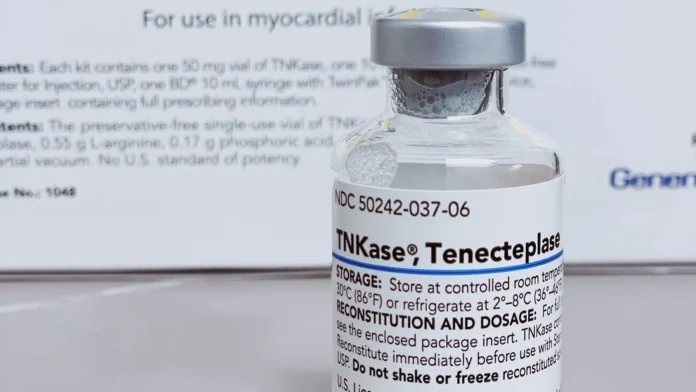
Genentech, a Roche subsidiary, announced that the U.S. Food and Drug Administration (FDA) has approved Tenecteplase, sold under the brand name TNKase, to break up blood clots in adults who have had an acute ischemic stroke (AIS).
TNKase is administered as a 5-second intravenous injection, offering a faster and easier alternative to the company’s Activase/alteplase, which requires a 60-minute infusion.
The drug is a laboratory-made version of the natural protein tPA, which helps break up blood clots, and is designed to target clots better and last longer than tPA.
Stroke is the fifth leading cause of death and disability in the United States, affecting nearly 800,000 people each year. AIS occurs when a blood clot blocks blood flow to the brain, leading to brain damage, disability, or death.