Inclisiran Receives FDA Approval for New Indication to Treat Hypercholesterolemia
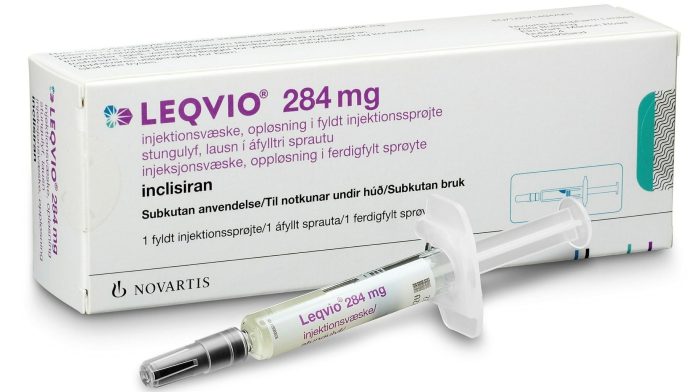
The FDA has approved a label update for inclisiran (Leqvio; Novartis Pharmaceuticals), allowing its use as monotherapy, in addition to diet and exercise, to reduce low-density lipoprotein cholesterol (LDL-C) in adults with hypercholesterolemia.
This first-line label update reinforces [inclisiran’s] proven ability to effectively lower LDL-C, a critical risk factor for heart disease,” Victor Bultó, president of US Novartis, said in a news release.
Inclisiran’s Indications and Approval
Inclisiran was originally approved by the FDA in December 2021 as an injection with diet and maximally tolerated statin therapy for adults with heterozygous familial hypercholesterolemia (HeFH) or clinical atherosclerotic cardiovascular disease (ASCVD). It is indicated as a 284-mg dose administered under the skin, with a second dose at 3 months and continued treatment once every 6 months.
The effectiveness of inclisiran was studied in 3 randomized, double-blind, placebo-controlled trials that included a total of 3457 individuals with HeFH or clinical ASCVD. The primary measure of effectiveness across all studies was the percentage change in LDL-C from the start of the trial to day 510. Across all 3 studies, individuals received subcutaneous injections of either 284 mg of inclisiran or a placebo on days 1, 90, 270, and 450.
A total of 1561 adults with ASCVD were included in study 1. By day 510, individuals receiving inclisiran experienced an average LDL-C decrease of about 51%, whereas the placebo group demonstrated an average increase of 1%.
In study 2, 1414 individuals with ASCVD were included, and at day 510, the inclisiran group’s LDL-C decreased by an average of 46%, compared with an average increase of 4% in the placebo group.
Study 3 included 482 individuals with HeFH. By day 510, individuals who were treated with inclisiran showed an average LDL-C decrease of 40%, whereas the placebo group’s LDL-C increased by an average of 8%.
The approved label change follows the FDA request for an update due to positive LDL-C lowering data for proprotein convertase subtilisin/kexin type 9 (PCSK9)-targeting therapies.
PCSK9 is a novel therapeutic target to lower LDL-C levels and reduce cardiovascular risk. Medications like inclisiran inhibit PCSK9, which in turn increases the availability of LDL receptors in liver cells, leading to a reduction in LDL-C levels.
Atherosclerotic Cardiovascular Disease and Hypercholesterolemia
ASCVD accounts for 85% of all cardiovascular deaths and is reported to have a greater burden than any other chronic disease in the United States. The disease is caused by the development and growth of atherosclerotic plaque in the inner lining of the arteries, which mainly includes LDL-C that collects over time. Increasing exposure to LCL-C raises the risk of cardiovascular events, including heart attack or stroke.
Hypercholesterolemia is the disorder known for excess of LDL in the blood, commonly affecting individuals older than 40, women, and women who are postmenopausal. The condition is very common, impacting about 1 in 20 individuals. Most individuals with hypercholesterolemia do not experience symptoms; however, individuals with severe disease may have cholesterol deposits on the eyelid or connective tissue.
As an injectable prescription medication, inclisiran improves hypercholesterolemia outcomes and is indicated as an adjunct to diet and exercise to reduce LDL-C in adults with hypercholesterolemia, in addition to HeFH.
“With this new indication enabling [inclisiran’s] use as monotherapy along with diet and exercise, we now have the potential to help even more patients achieve their LDL-C lowering goals earlier in their treatment journey,” Bultó said in the news release.