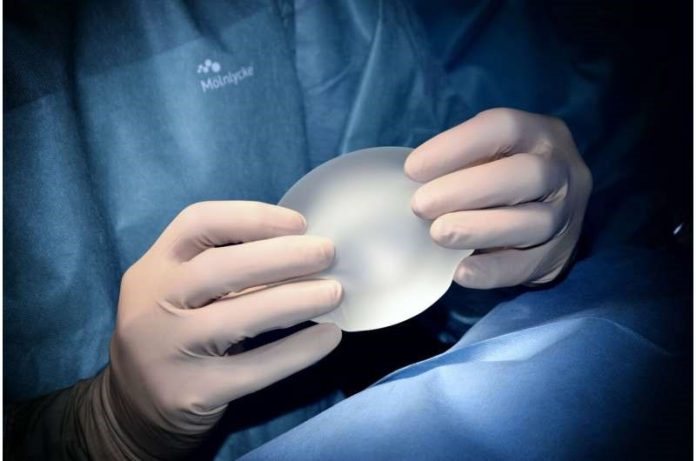
Implant-based reconstruction linked to increased risk of breast lymphomas
Columbia University investigators report an increased risk of breast lymphomas after postmastectomy implant-based reconstruction, including anaplastic large-cell lymphoma and several B-cell and T-cell non-Hodgkin lymphoma histologies.
Previous work has described an increase in anaplastic large-cell lymphomas in association with breast implants. Adverse events resulted in a black-box warning by the US Food and Drug Administration on all breast implants.
In 2022, the FDA issued a public safety communication expanding these observations to include various non-Hodgkin lymphomas. Observation rates were limited to case reports linking implants to anaplastic large-cell lymphoma.
Implant-associated anaplastic large-cell lymphoma has been attributed to chronic inflammation that facilitates lymphoproliferation and malignant transformation within a hypoxic tumor microenvironment. Similar causes may contribute to the pathogenesis of other non-Hodgkin lymphomas, including B-cell lymphomas.
In the study, “Lymphomas of the Breast After Postmastectomy Implant-Based Breast Reconstruction,” published in JAMA Network Open, researchers identified women who underwent postmastectomy implant-based reconstruction to report the risk of lymphomas of the breast.
Investigators identified 61,043 women who underwent postmastectomy implant-based reconstruction for any breast tumor with a median age of 51 years (44–60) and a median follow-up of 86 months (49–133), with 478,864 person-years observed. Race and ethnicity included 219 American Indian or Alaska Native, 4,565 Asian or Pacific Islander, 4,941 Black, 6,227 Hispanic, 44,947 white, and 144 unknown.
Participants were followed for pathologically confirmed lymphomas until death, loss to follow-up, or end of study, with a latency exclusion period of two months from the primary breast cancer diagnosis. Multiple primary-standardized incidence ratios compared observed vs. expected cases based on incidence rates from the US female population, adjusted for age, race and ethnicity, and year of diagnosis.
Across follow-up, 15 non-Hodgkin lymphomas of the breast occurred with a standardized incidence ratio (SIR) of 5.03, or five times the expected rate. Seven were anaplastic large-cell lymphomas with a SIR of 41.6 (~42 times the expected rate) and eight were other histologies with a SIR of 2.84.
Diagnoses included five diffuse large B-cell lymphomas (SIR, 5.26), two small lymphocytic lymphomas (SIR, 16.7), and one peripheral T-cell lymphoma, not otherwise specified (SIR, 11.8, or nearly 12 times the expected rate).
Excess risks per 1,000,000 persons per year measured 8.5 for diffuse large B-cell lymphoma, 3.9 small lymphocytic lymphoma, and 1.9 peripheral T-cell lymphoma not otherwise specified.
No increased risk of non-Hodgkin lymphoma outside the breast or Hodgkin lymphoma of the breast was observed. Risk of non-Hodgkin lymphoma of the breast was also not increased in women who received mastectomy without immediate implant-based reconstruction or lumpectomy with or without radiotherapy.
The authors report a novel epidemiologic association of breast implants with both B-cell and T-cell non-Hodgkin lymphomas, with increased risk observed for diffuse large B-cell lymphoma, small lymphocytic lymphoma, and peripheral T-cell lymphoma, not otherwise specified, in addition to anaplastic large-cell lymphoma.
It is important to appreciate that the absolute risk of lymphoma is extremely low and similar for anaplastic large-cell lymphoma and other non-Hodgkin lymphoma histologies. The authors state that they have not identified an elevated risk of breast squamous cell carcinoma following implant-based breast reconstruction.
The FDA is aware of fewer than 30 cases of non-anaplastic large-cell lymphomas in breast implant capsules vs. over 1,300 cases of anaplastic large-cell lymphoma. Continued surveillance of breast implant–associated malignant neoplasms is warranted by government and regulatory agencies.
Limitations include inability to evaluate women who underwent cosmetic implantation or noncancer-directed surgical procedures in the contralateral breast. Future studies should further investigate patient and implant-specific risk factors.