BrainSense brain stimulator approved for Parkinson’s patients
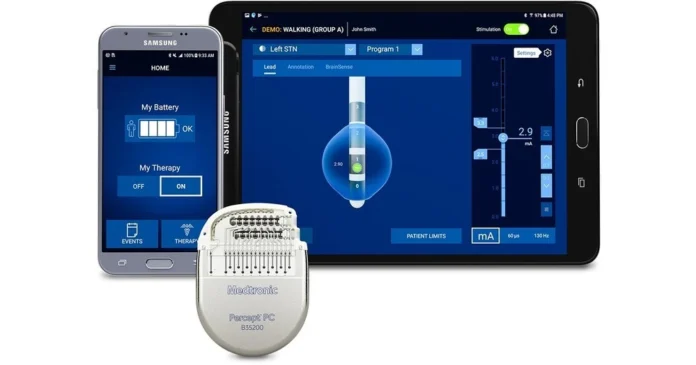
Medical device maker Medtronic announced Monday that the U.S. Food and Drug Administration (FDA) has approved the company’s BrainSense adaptive deep brain stimulation (aDBS) device for use in patients with Parkinson’s disease.
The approval comes just one month after Medtronic received a CE mark in Europe for a device that Medtronic designed to automatically adjust stimulation for Parkinson’s patients based on their unique brain activity to address symptoms such as involuntary tremors and balance problems.
The company also received FDA approval for the BrainSense device’s Electrode Identifier (EI). EI, also approved in Europe, provides a detailed view of the brain signals of Parkinson’s patients to help doctors better plan deep brain stimulation (DBS) and optimize treatment.