Neffy nasal spray approved for severe allergic reactions
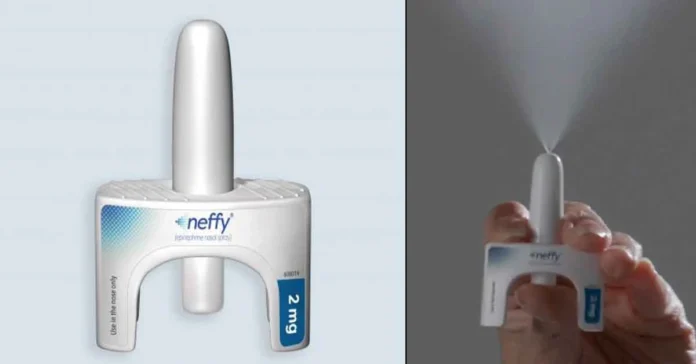
ARS Pharmaceuticals announced Wednesday that the U.S. Food and Drug Administration (FDA) has approved a 1-milligram dose of the company’s nasal spray, brand name Neffy, for severe allergic reactions in patients weighing between 15 and 30 kilograms.
The 2-milligram dose of the drug was previously approved for people weighing more than 30 kilograms.
Neffy, which was first approved by the FDA in August 2024, is the first needle-free emergency treatment for potentially fatal allergic reactions, and an alternative to the EpiPen and other auto-injector devices that are filled with epinephrine.
The drug is designed to be administered at the first sign of a severe allergic reaction to prevent life-threatening situations such as anaphylaxis. Anaphylaxis is a severe allergic reaction that can occur within seconds of exposure to an allergen.