FDA warns unapproved hair-loss product finasteride tied to side effects
A quick spray of medication might seem like an easy way to get thicker hair, but some people say one sold online has left them battling sexual side effects, depression and even thoughts of suicide.
Now, the U.S. Food and Drug Administration is warning the public about the risks.
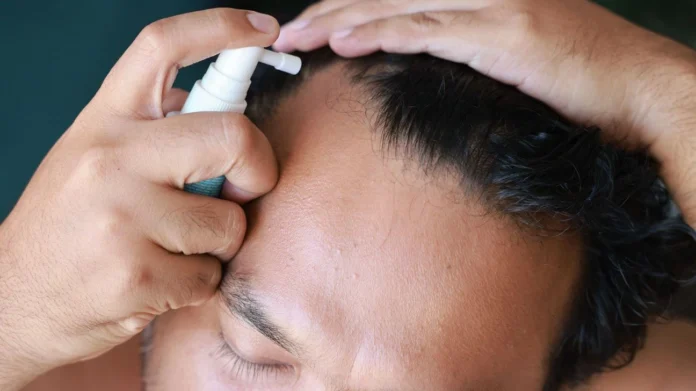
The FDA warning is about a topical spray version of finasteride, a drug often sold online by telehealth companies like Hims, Keeps and Ro, The Wall Street Journal reported.
While the pill form of finasteride, known by the brand name Propecia, is FDA-approved to treat hair loss, the spray version is not.
“FDA has become aware of reports of adverse events involving compounded topical finasteride products potentially putting consumers at risk,” the April 22 statement said.
“Currently, there is no FDA-approved topical formulation of finasteride,” it continued. “Compounded topical finasteride products do not have FDA-approved labeling. There are potential serious risks associated with the use of compounded topical finasteride products.”
The FDA warning follows a recent Wall Street Journal report describing men who said they experienced side effects after using finasteride prescriptions purchased from the telehealth companies Hims and Keeps.
Reported side effects include “erectile dysfunction, anxiety, suicidal ideation, brain fog, depression, fatigue, insomnia, decreased libido and testicular pain,” the FDA said.
The FDA also warned that the spray could pose a risk to others, especially women. Finasteride can cause birth defects in male babies, so women are advised not to come in contact with the drug, the warning added.
Some users told the FDA that their doctors had said the spray version would not cause side effects.
Online health companies are allowed to sell these sprays through compounding pharmacies, which create drugs when patients need an alternative to FDA-approved medications.
While it’s all legal, these companies are not held to the same advertising standards as traditional drugmakers, The Wall Street Journal reports.
In previous statements, both Hims and Keeps said they inform customers about potential risks on their websites and packaging.
The FDA also warned that the spray could pose a risk to others, especially women. Finasteride can cause birth defects in male babies, so women are advised not to come in contact with the drug, the warning added.
Some users told the FDA that their doctors had said the spray version would not cause side effects.
Online health companies are allowed to sell these sprays through compounding pharmacies, which create drugs when patients need an alternative to FDA-approved medications.
While it’s all legal, these companies are not held to the same advertising standards as traditional drugmakers, The Wall Street Journal reports.
In previous statements, both Hims and Keeps said they inform customers about potential risks on their websites and packaging.