FDA OKs New Drug for Rare Head and Neck Cancer
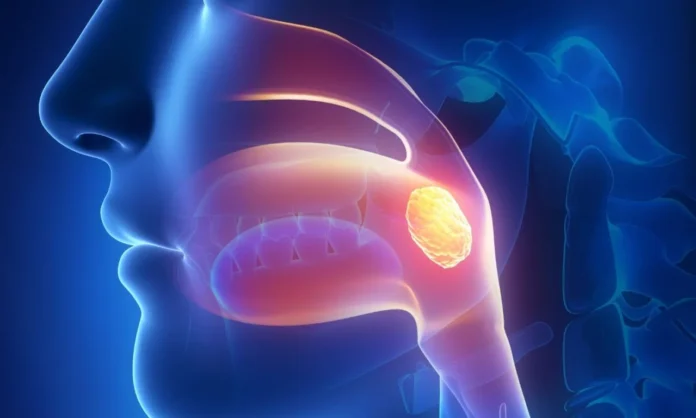
The FDA has approved a new drug for adults with a rare type of head and neck cancer known as non-keratinizing nasopharyngeal carcinoma (NPC) after studies showed that patients treated with the drug lived longer without their cancer worsening than with standard treatments.
The drug, known as penpulimab, is meant for advanced NPC that has come back or spread. It can be used with standard chemotherapy as the first treatment choice or used alone if patients have already tried other treatments.
NPC starts in the upper part of the throat, behind the nose, in an area called the nasopharynx. About 133,000 new cases are diagnosed globally each year, according to estimates from the World Health Organization. About 70% of these cases are found at an advanced stage, which can make treatment more challenging and increase the risk of the cancer returning.
“This milestone enhances international treatment guidelines for advanced NPC and extends the benefits of China’s innovations to global patients, ultimately reshaping the treatment landscape for metastatic NPC worldwide, Chaosu Hu, PhD, of the Fudan University Shanghai Cancer Center and principal investigator of the clinical trials, said in a press release from Akeso, the drug’s maker.
The FDA’s decision was based on clinical trials showing penpulimab works better than standard chemotherapy treatments in patients with advanced NPC. In a study involving 291 patients who had not received prior chemotherapy, participants were randomly assigned to receive either penpulimab or a placebo, both combined with standard chemotherapy. Results showed that compared with the placebo combo, the penpulimab combination helped delay cancer progression (9.6 months vs 7 months) and kept more patients (31% vs 11%) alive without their cancer worsening after one year of follow-up. Another study followed patients with NPC whose cancer had progressed despite previous treatments. In this trial, 28% of patients receiving penpulimab alone experienced benefits.
Penpulimab is a lab-made antibody that helps the immune system fight cancer. It works by blocking PD-1, a protein on cancer cells that promotes their growth and spread by helping them to hide from the immune system. Patients with NPC have high levels of PD-1, making it a good target for treatment. Penpulimab is given via IV once every three weeks when used with chemotherapy, or once every two weeks if used alone, for up to two years depending on the patient’s condition.
The most common side effects when penpulimab is used with chemotherapy include nausea, vomiting, low thyroid function, constipation, low appetite, weight loss, cough, COVID-19 infection, tiredness, rash, and fever. When taken alone, the most common side effects are low thyroid function and joint or muscle pain. Patients may also experience immune-related side effects such as lung inflammation, liver or bowel problems, hormonal issues, kidney trouble, and skin reactions.