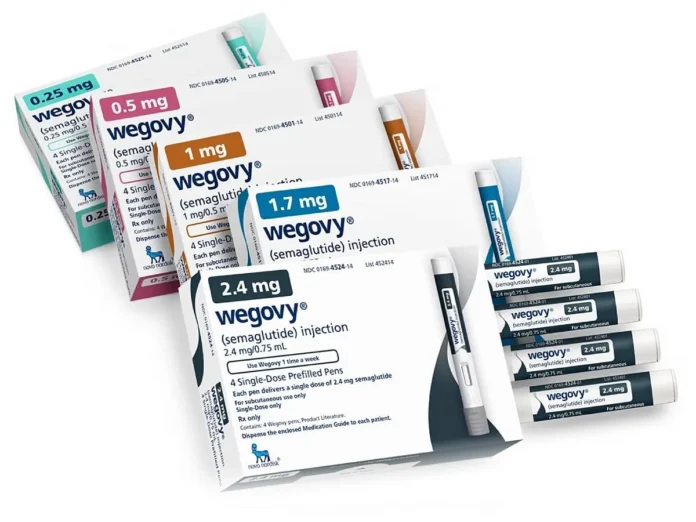
Novo Nordisk’s obesity drug Wegovy debuts in Thailand
Novo Nordisk (NOVOb.CO), opens new tab has launched its hugely popular weight-loss drug Wegovy in Thailand, an executive of the Danish drugmaker’s local subsidiary said on Monday, marking the injectable drug’s first entry into the Southeast Asian market.
First launched in 2021, Wegovy helped make Novo Nordisk Europe’s most valuable listed company until recently, worth $615 billion at its peak.
Wegovy is available in more than a dozen countries including the United States, Japan and China, with Thailand becoming its latest market.
“We actually received the Thai FDA approval already in 2023,” said Enrico Canal Bruland, vice president and general manager of Novo’s Thai subsidiary. He noted that Novo was making Wegovy available in Thailand ahead of rival Eli Lilly‘s (LLY.N), opens new tab Zepbound weight loss drug.
Bruland declined to provide details on Wegovy’s pricing in Thailand, which has a population of around 66 million, or Novo Nordisk’s plans for expansion into other Southeast Asian markets.
Wegovy is currently available for prescription in private hospitals around the country and will be available soon in public hospitals, Bruland said.
“Over the last four years, we have invested approximately 500 million Thai baht in clinical trials in Thailand,” he said.
Novo Nordisk’s diabetes drug Ozempic, which contains the same active ingredient as Wegovy, is already available in Thailand.
About 42% of Thailand’s adult population is considered obese, and the rate of obesity in school children surged from 5.8% to 15% within two decades, according to data from the country’s health ministry.
“If we then look at the economic impact that this has, approximately 1 percent of GDP is used for health-related costs that come with obesity and productivity loss,” Bruland said.
“We believe with this innovation, we can make a big difference and hopefully bend this curve.”