Weight-Loss Pill Approval Set to Accelerate Food Industry Product Overhauls
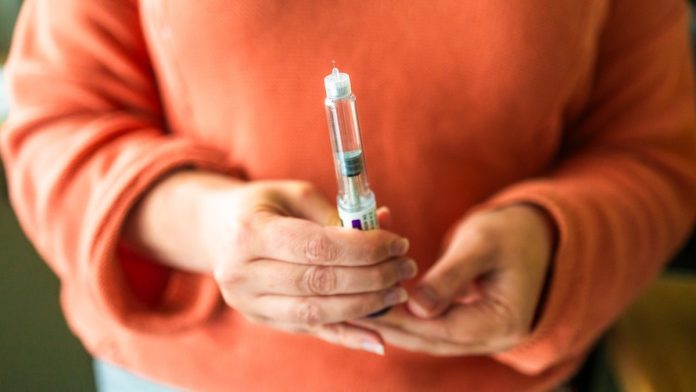
Packaged food makers and fast-food restaurants may be forced to overhaul more of their products next year as newly approved, appetite-suppressing GLP-1 pills become available in January, analysts say.
More Americans are expected to try the drugs as a pill rather than as a shot because the medication will be cheaper and many patients are hesitant to inject themselves.
The U.S. Food and Drug Administration approved Novo Nordisk’s Wegovy GLP-1 pill on Monday, sending shares of food companies down on Tuesday. Eli Lilly’s rival medication is expected to gain approval from regulators next year.
Food companies including Conagra Brands and Nestle are already dealing with shifts in consumer tastes toward higher protein and smaller portions due to the popularity of weight-loss injections, and analysts believe widespread GLP-1 adoption could mean long-term changes in demand.
To cope, businesses are promoting products with more protein, tweaking labeling to say they are GLP-1 friendly and working with large retailers to better market products.
“We are seeing people cut (back) specifically on salty snacks, liquor, soda, drinks, and bakery snacks, and more focused on protein and fiber, so we expect food companies and also restaurants to cater to this audience that is growing,” said JP Frossard, consumer foods analyst at Rabobank.
“We’ll see more access to those drugs and a higher addressable market for products that have in mind the needs of the GLP-1 user,” he said.
Andrew Rocco, stock strategist at Zacks Investment Research, called Novo’s approval “groundbreaking” because the pill would be cheaper than the injectable version of Wegovy and deliver the same weight-loss metrics. “High protein, smaller portions, and functional food innovation will be necessary,” he said.